Category Archives: Cystic Fibrosis
Cystic Fibrosis Notes and Notable: Jeanne Barnett on Patient Communities – What They Do and How They Help
 Drug Market Info presents Cystic Fibrosis Notes & Notable, an interview series with leading voices in the struggle against Cystic Fibrosis. (For more data on Cystic Fibrosis, see the Drug Market Info Cystic Fibrosis Fact Sheet)
Drug Market Info presents Cystic Fibrosis Notes & Notable, an interview series with leading voices in the struggle against Cystic Fibrosis. (For more data on Cystic Fibrosis, see the Drug Market Info Cystic Fibrosis Fact Sheet)
Today we present our interview with Jeanne Barnett, a teacher, web developer and patient advocate who established CysticFibrosis.com. She established CysticFibrosis.com in 1996 through a company called Medrise. Medrise develops solutions for online media, market research and health resources for various audiences.
CysticFibrosis.com spawned a new model of information exchange, at least in part because cystic fibrosis (CF) patients are often quarantined from each other to avoid bacterial infection. However, CF patients also undergo very complex, time-consuming treatments, which can be greatly improved through interaction with others. CysticFibrosis.com has provided the CF community with a way to connect to each other and has evolved into a vibrant online social network. Jeanne also advises and coaches companies on how to engage with the CysticFibrosis.com e-patient community.
We caught up with Jeanne recently to learn more about this one-of-a-kind resource. Here is what Jeanne has to say about CysticFibrosis.com:
You have a very interesting background in online media. How did you get involved with cystic fibrosis?
The site was originally designed because my partner had CF. It became instantly busy as patients reached out, understood and empathized with each other. As I reviewed the site in this early stage, I began to realize it was becoming layered with stories and facts about the disease itself. I was so taken by the huge unmet need that I decided to commit for the long haul.
CysticFibrosis.com has evolved into patients from all over the world sharing their experiences with drugs, delivery systems, the devices and equipment they are using, exercise regimens, alternative medicine, diet, treatment protocols as well as their specific CF mutations. Members have a deep-rooted need for belonging and knowing more in order to have hope in the future.
How many members are in the online community and where are patients located?
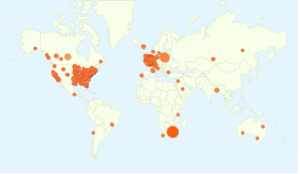
We have 14,000 members who have filled out profiles, many including optional genetic mutation information; over 11,000 are in the US and close to 700 in the UK. Overall, we have 121 countries and territories represented.
Who exactly is the community composed of…is it mostly patients, caregivers or others?
We have 40% patients, 45% caregivers and 15% others.
Do you need to be member to access the site and what does membership entail?
You don’t have to be a member to read the messages. The site is free to all and about 2,000 viewers access it every day. Membership entails filling out a form with some optional questions about research and mutations. Once you are a member, you can receive our newsletters and you can post on the site.
Is the community open to industry and what’s the best way for a company with CF products to interact?
Our site facilitates and is an agent for CF patients. In addition, we have good relationships with many pharmaceutical companies and device manufacturers. The best way for a company to interact is to address patients’ needs and answer questions patients have. Companies have done this in the past with general helpful videos. Patients have also signed up for clinical trials through our site. Companies can ask questions of our community and we have excellent response rates to our surveys, usually 500 members in 3 days.
What do you view as the primary benefit of cysticfibrosis.com for the CF community?
CysticFibrosis.com saves lives. I have heard this in testimonials many times. Perhaps, people have lost hope. By sharing experiences, people find new ways to cope with a challenging condition. Or it may be that patients have never heard of a particular treatment or how important exercise and diet are to their well-being. Our community serves as a backup means of support and caring.
What drives your members to continue returning and contributing?
This is a complex disease and members learn something new every day. Patients can easily be on 17 drugs at a time and use three or more medical devices. There are always updates, new products, and clinical trials on the forefront. Besides an abundance of empathy, there is a lot of knowledge about the disease. CysticFibrosis.com is easily found on the web and may be one of the first places a new patient or caregiver finds on a Google search.
What predominant themes or issues have emerged since you started the site?
Patients talk about medications, delivery systems, activities of daily living, how to sanitize and clean equipment (always an ongoing question) because manufacturer’s directions may be inconsistent with established patient procedures.
Even when people branch out on their own, starting a site or blog, they stay tethered to our community.
Do you routinely survey the CF community? How do you like to work with companies that have products for CF patients?
Yes, we routinely survey, and the community always responds to surveys. Patients like to contribute important information. In this way they can stay informed and learn what is on the horizon in the way of drugs, devices, and healthier living. I suggest always allowing this community to comment on surveys. We have so much to learn from them!
They are the experts (In The Outliers, Malcolm Gladwell defines an expert as someone who has studied a topic for 10,000 hours). These patients are way over that and converse on so many levels about their disease and living with it.
How do you see CysticFibrosis.com evolving?
We have set up patient retail in the last few months on our Medrise.com site. This is a store where patients with cystic fibrosis can find many of the products and devices they use every day, as well as interact with manufacturers of CF products. We are also in the process of initiating a Pilot study to learn about Home Spirometry and CF patients.
Concerning genetics and the Human Genome Project, Cystic Fibrosis patients are aware of their mutations. They often self group by mutation, accordingly on our site. We are moving to help them form guilds based on their mutations. From there, they can map their genomes, compare and contrast disease progression, discuss their treatments with each other and their Health Care Providers and work directly with researchers.
Mary Beth Cicero: Why I’m Walking in the Great Strides Walk for Cystic Fibrosis May 20
May is Cystic Fibrosis Awareness Month and one of the largest national fundraising events takes place this Sunday. It is the Great Strides Walk, which is taking place in over 200 cities this Saturday and Sunday, May 19-20. (Click to find out more about Great Strides, including walk locations.)
I will actually be walking twice! Last year when Drug Market Info was looking for CF patients to interview for our Cystic Fibrosis Market Info report, I contacted my good friend Sue (whom I hadn’t seen in years) but knew her daughter had CF. We ended up interviewing her and a number of her friends from western Massachusetts and were completely touched by their stories.
Our interviews with these patients and caregivers took place last November and they mentioned the Great Strides Walk to me. I promised them that I would be there and so here it is May and I am on my way to both Northampton and Shelburne Falls Mass. I will be supporting Team Paige and Audrey’s Angels.
I am so excited because I will be meeting some of the patients and mothers we interviewed that contributed so many insights to our Cystic Fibrosis Market Info report. I truly hope that the patients’ perspectives they shared will influence companies to either undertake further drug development on Cystic Fibrosis or take their needs into account. (You can get the facts on Cystic Fibrosis here.)
I am particularly excited to meet in person one of the patients we talked with that has had 2 lung transplants—she touched my heart and I can’t wait to meet her! When I asked her about her goals in life she said she didn’t have any. Her mother interjected that she doesn’t know how long she will live and doesn’t like to think about the future. Of course, I jumped in and said, “Why don’t you want to be the first person to have 3 lung transplants!” I am hoping that won’t have to happen and that she will thrive and be happy.
She did mention that she always wanted to go skydiving. Even though I want to make her happy… I am drawing the line at skydiving! However, I am going to take her zip lining and I am happy to report that Bob Coughlin (President of Mass BIO, read our Notes & Notable interview with Bob Coughlin) who has a son with CF is not as “chicken” as me and has graciously agreed to take her sky diving.
To all of you out there with CF and to those who love you, as well as all of the drug researchers trying to cure this disease—I salute you and will be proudly walking alongside of you on Sunday.
Cystic Fibrosis Notes and Notable – Dr. Brian O’Sullivan, Cystic Fibrosis Center Director, UMass Memorial Medical Center
 Drug Market Info presents Cystic Fibrosis Notes & Notable, an interview series with leading voices in the fight to cure Cystic Fibrosis.
Drug Market Info presents Cystic Fibrosis Notes & Notable, an interview series with leading voices in the fight to cure Cystic Fibrosis.
Today’s interview is with Dr. Brian O’Sullivan, Cystic Fibrosis Center Director and Professor of Pediatrics at the University of Massachusetts Medical School. Dr. O’Sullivan, who has been working with CF since 1987, was asked to comment on recent developments in the treatment of Cystic Fibrosis. Here are his comments:
What are the biggest hurdles to treating CF?
The single biggest hurdle is patient adherence to medication usage. We ask them to do so much and many of the therapies are very time consuming (nebulizers and chest physical therapy). It is hard for patients to do all that they should.
What are the top advances that you have seen in last five years for CF patients and what is on the horizon?
Kalydeco (recently launched) and the other Vertex compound (VX-809) in clinical development are the top advances. Hopefully other approaches to the basic CF defect will continue to be developed in the next five years for Cystic Fibrosis. I am also looking forward to PTC Therapeutics Ataluran (PTC-124) Phase 3 trial results. Gene therapy studies will be important going forward.
In your opinion, what are the major benefits and drawbacks of Vertex’s recently approved Kalydeco?
The major drawback for Kalydeco is the limited applicability. FDA approval is needed for all Type 3 mutations, not just G551D. The cost ($300,000 a year) is just too much for patients and insurers to swallow. Obviously, if more patients are eligible to use it the cost can come down.
Do most CF patients already know their specific CF mutation and when is this genetic testing usually performed?
Genetic testing has become the norm, especially with newborn screening algorithms that include it. A lot of patients do not know their specific mutations off the top of their heads but they know to ask about them.
Can you tell us a little more about the G551D mutation? Is it hard to detect?
G551D is a mutation which is included on all newborn screening and OB/GYN screening panels, thus it is not hard to detect.
In general, are CF patients with the G551D mutation affected more or less severely than those with other mutations or are they about the same?
G551D is a class 3 mutation. This class is considered to be severe since it leads to loss of function of the CFTR protein.
How will Kalydeco change CF treatment in those patients qualified to use it? Do you believe that Kalydeco could have an impact on CF life span?
I believe Kalydeco will increase CF lifespan. Unfortunately, we still do not have enough data on potential side effects. Until it is used in thousands of patients for several years, we will not know the full consequences (for better and for worse) of its action. If it is as good as we hope, it could lead to decreased need for other therapies, which would offset its cost somewhat. The pivotal study involved subjects continuing their usual medical routine, so we have no idea if the benefits seen with it will be present in the absence of other routine CF care.
The mutation occurs in about approximately 1,200 people in the US or 4% with CF, so can you try to quantify the impact that Kalydeco will have on treating the disease as a whole?
We are nibbling at the edges. Kalydeco will not affect the vast majority of CF patients. However, it does provide hope for others that their mutations may too succumb to small molecule therapy.
About Dr. Brian O’Sullivan
Dr. O’Sullivan is a pediatric pulmonologist and the Cystic Fibrosis Center Director at UMass Memorial Medical Center in Worcester, MA. He has served as Chair of the UMass Medical School IRB for the past 10 years. He co-chaired the pulmonary care guidelines committee for the National Cystic Fibrosis Foundation and has served on their Center Directors Committee.
Looking for the facts about Cystic Fibrosis and the Cystic Fibrosis market? The following information comes from our Cystic Fibrosis Market Info™ Report which is now available for purchase and download on our website, Drug Market Info.
Cystic Fibrosis Notes and Notable – Robert K. Coughlin, President & CEO, Massachusetts Biotechnology Council
Drug Market Info presents Cystic Fibrosis Notes & Notable, an interview series with leading voices in the fight to cure Cystic Fibrosis.
Today’s interview is with Robert K. Coughlin, the President & CEO of Massachusetts Biotechnology Council and the father of a son with Cystic Fibrosis. Bob is a major figure in the biotech community, a devoted father and major fundraiser for Cystic Fibrosis. He was asked to share his personal experiences and provide a perspective on drug development. Here are his comments:
Can you tell us a little bit about your son’s experience with CF and your family’s experience as caregivers?
Bobby is our third child and is currently nine years old. Our older two children were healthy and thriving. We found out in utero (21 weeks into the pregnancy) that he was affected with Cystic Fibrosis. Mom was screened for the mutation for this pregnancy and not the prior two. When we found out Mom was a carrier, I was screened and also found to be a carrier of the CF mutation. We then proceeded to test our unborn child through DNA testing of the amniotic fluid. We learned we both passed off the mutation and he would be born with CF. This knowledge enabled us to treat the disease immediately. We had the opportunity to grieve prior to him joining us and we have had a positive attitude since the beginning.
What are the daily struggles and triumphs? How do you measure or evaluate his health/progress?
Bobby’s life is not easy. He takes many meds, has trouble digesting food and struggles to gain weight. He is a very outgoing little boy that doesn’t let it hold him back. He plays hockey and baseball and loves to stay active. Overall he has been relatively healthy for a CF patient. He has only been hospitalized once for a clean out and most of his therapies we can administer at home.
On a typical day, how many medications does your son use? How much time does this involve out of his day?
Bobby often says that the worst part about having CF is the amount of time he needs to spend taking nebulized meds and receiving chest physical therapy. He gets chest PT three to five days per week. These sessions take approximately an hour for the therapist to manually “clap” various areas of his chest to loosen mucous from the lungs. He also needs to take two to three nebulized meds per day and each session takes from 30 to 45 minutes. He gets really sad sometimes when other kids are outside playing and he needs to dedicate this time to staying healthy. It is difficult for a 9-year-old, active boy to understand this but he mostly stays positive.
He has to take 3-5 pancreatic enzyme pills with every meal and snack. Some days he takes up to 50 pills per day just to eat food. These enzymes don’t work well much of the time and he has very uncomfortable abdominal pains. He also takes vitamins A, D, E, C orally as well as some other supplements. Lastly, he is on a medicine that he takes twice a day to help keep the swelling of his liver under control.
What improvements are needed in these therapies that might make them easier to take?
As parents and advocates, we always encourage the formulation of both better drugs and more efficient delivery modes. Bobby would love nebulized meds to be done “really fast” so he can get out and play. Living life is important to anyone facing chronic illness. The only pancreatic enzymes available are animal-based and deteriorate over time due to temperature changes. There needs to be a synthetic solution that can be dosed in a way to reduce the pills needed every day.
What are your thoughts on the current state of CF therapies available to patients?
We have decided to continue with manual chest PT as opposed to using a mechanical vest for this therapy. We feel that the current vests are not as effective. Until recently, most of the CF medicines were antibiotics to fight infection in the lungs. This is not a sustainable treatment due to the damage caused during infection and the bacteria’s ability to build up a resistance and stop working as the patient gets older. We have desired for many years that a synthetic enzyme be invented as well as a therapy that would fix the defect as opposed to treating the symptoms of the disease.
Do you know what CF mutation your son has? If yes, when/how did you learn of this?
My son has the deltaF508 mutation from me and the 1717-1 from his Mom. We learned of this before he was born.
How will the recent approval of Kalydeco (Vertex) change the treatment of CF for those who are able to use it?
The approval of Kalydeco is going to and is currently changing the world for patients with the G551D mutation. They may no longer be required to take all of the other therapies and hopefully the physical deterioration will be stopped in its tracks! The work is not done and we strongly support and advocate for the follow-on products that will enable Kalydeco to work for all of the CF mutations.
Do you think there are enough companies pursuing drug development for CF?
For rare diseases, there is often not enough research being done. We could argue that if there is an unmet medical need, you need to do more and more research. However, I feel that the Cystic Fibrosis Foundation has rallied with the Cystic Fibrosis community for many years to raise both awareness and research dollars. We have been and always will continue to raise money and ultimately find a cure for all who suffer from this horrible disease.
About Robert Coughlin
Bob Coughlin is President and CEO of Massachusetts Biotechnology Council. He has spent his career in both the public and private sectors, most recently serving as Undersecretary of Economic Development within Massachusetts Governor Deval Patrick’s administration. He was elected as State Representative for three terms where both healthcare and economic development were his priorities.
Bob has served as the honorary chairman of the Great Strides Cystic Fibrosis Walk and has co-chaired the Children’s Hospital Boston signature event, Champions for Children’s. His son Bobby has Cystic Fibrosis and is 9 years old.
Looking for the facts about Cystic Fibrosis and the Cystic Fibrosis market? The following information comes from our Cystic Fibrosis Market Info™ Report which is now available for purchase and download on our website, Drug Market Info.
Perfect Timing: Vertex Cystic Fibrosis Drug Approved and Cystic Fibrosis Market Info Report Now Available
Our Cystic Fibrosis Market Info™ Report, Drug Market Info’s newest market research report, is perfectly timed for analysts and drug developers to assess the impact of Vertex‘s new ground-breaking therapy for Cystic Fibrosis (CF), Kalydeco. It provides explicit disease information, the current drug armamentarium for CF, the five-year trend for the CF market value, detailed pricing and pertinent data on the CF pipeline. In addition, extensive patient input (our signature “patients’ perspective“) offers a glimpse into how CF and its treatment impacts lives. All of this supports our conclusion that the FDA approval of Vertex’s Kalydeco is a major step forward in the treatment of Cystic Fibrosis (CF) because…
- It validates genetic therapy — it is the first step toward a genetic cure for a disease caused by a faulty gene
- It treats the causes, not just the symptoms of the disease: First time ever for a CF therapy
- It could lower the high treatment burden of CF: Potentially decreases the medication load versus adding more to the pile
- It underscores the importance of collaborative agreements with the Cystic Fibrosis Foundation in this area: Kalydeco was discovered through a research collaboration with Vertex and the Cystic Fibrosis Foundation Therapeutics, Inc. (CFFT)
Maybe most importantly, this paves the way for a new research and drug development approach for any genetic disorder. Kudos to Vertex!
Sue Casarona,
Vice President,
Drug Market Info
Why do a Market Info Report on Cystic Fibrosis?
With all of the therapeutic areas where we could focus, I guess the natural question comes up why Drug Market Info would do a report on cystic fibrosis (CF). After all, the big markets like diabetes, heart disease, and cancer could be easily covered and would appeal to a larger audience. Cystic Fibrosis in this realm is tiny. With only 30,000 patients it would almost appear insignificant and falls well below the threshold of what is considered an orphan disease (<200,000 patients). Once again, we ended up being fascinated with this disease. For one thing it touched us personally. One of my best friends from college died of CF. She was a spitfire and truly lived each day as if it counted and…it did.
We also had done some work in this area by trying to help a start-up biotech company get prepared for the launch of their first drug which was for cystic fibrosis. We, of course, became totally intrigued. The disease is challenging, heart wrenching and so far incurable. The patients and their families have an indomitable spirit that we have never seen the likes of! What a beautiful (and handsome) group of patients—you can’t find a CF patient that isn’t totally adorable—both inside and out. We still don’t know or understand what makes these patients so attractive but all you need to do is look at some of the patient photographs in our report, Cystic Fibrosis Market Info™, to know what we say is true.
This disease is hopeful despite the fact that the life span of CF patients is short. The good news is they are living longer (now almost half live over the age of 18) and there are at least 30 drugs being tested in the clinic for CF. Not bad for a tiny orphan disease. Each CF patient has their own story to tell and each is as different as they can be. We talked to a father of 2 CF children, not that different in age, but they both were on each end of the spectrum as far as how their disease affected them. We want companies developing drugs to understand this and at least have a peek inside what the CF patients think about the disease and what would make a difference in their lives. It’s why we make Patients’ Perspectives one of the three sections of each and every Drug Market Info Report.
The Cystic Fibrosis market is not well-studied. The last market report we could identify was published in mid-2010, which means it was probably written a year earlier. To our way of thinking this is dated information. At Drug Market Info we like to provide the most current full year market data and all of our information is sourced and cited with the latest references (and reviewed by a leading clinician). Markets change quickly and this market is set to explode with so many drugs in clinical trials and Vertex just filing one.
Again, as with some of our other reports, we believe this market is too often overlooked from a drug development perspective in the pulmonary area. Companies are going after COPD, asthma and all of the “big” indications. But wouldn’t it make sense to do something really wonderful and make it easy for a CF patient to breathe or have less risk of infection? Even the enzymes these patients take every day could be easily improved to make life and pill taking just little bit easier for them.
What do you think? Cystic Fibrosis Market Info is a thorough and extensive report that will provide all of the insights you need to understand this market. In addition, we have partnered with the Boomer Esiason Foundation—what a great organization that is making a difference for patients! Each report you buy generates a donation to them which will help CF patients.
Drug Market Info Launches Presentation-Ready Report on Cystic Fibrosis Market
Unique Cystic Fibrosis Market Info™ Report Focuses on Treatment of Orphan Disease from the Patients’ and Caregiver’s Perspective
NORWELL, Mass.–(BUSINESS WIRE)–Drug Market Info, a new Internet source for presentation-ready market reports with the Patients’ Perspective, announced today the launch of its newest research report — Cystic Fibrosis Market Info™. Cystic Fibrosis (CF) is an orphan indication that is a chronic, progressive, life-limiting autosomal recessive genetic disease that affects the lungs, digestive and reproductive systems.
“Cystic Fibrosis is a devastating disease that has, until recently, been predominantly a pediatric disease affecting tens of thousands of patients. Treatments are time consuming and costly, and the burden weighs heavily on both patients and caregivers”
There are about 30,000 patients, living with CF in the U.S., and while new treatments have added years to life expectancies, Cystic Fibrosis comes with a high treatment burden, which includes numerous and time-consuming daily medications and activities. To better understand the treatment of cystic fibrosis, as well as the market, pipeline and implications for future growth, Drug Market Info has launched a unique patient-focused report, “Cystic Fibrosis Market Info™.” This presentation-ready report is a comprehensive look at the CF market, including prescription data trended for five years. Cystic Fibrosis Market Info™ contains 90 customizable slides, 28 graphs and charts, photographs of patients and caregivers, with their insights and perspectives woven throughout. Drug Market Info’s CF report is the only market research report available today to provide patient/care-giver perspectives that highlight the struggles that are an unfortunate necessity of these daily, time-consuming treatment regimens. As with all Drug Market Info reports, Cystic Fibrosis Market Info™ was reviewed by a leading clinician in the field to provide the most current perspective on treatment and future trends.
“Cystic Fibrosis is a devastating disease that has, until recently, been predominantly a pediatric disease affecting tens of thousands of patients. Treatments are time consuming and costly, and the burden weighs heavily on both patients and caregivers,” said Mary Beth Cicero, Principal of Drug Market Info. “Drug Market Info is pleased to offer this report on a fascinating market that is not well-profiled and often overlooked from a drug development perspective. We hope that marketers will read and assimilate the perspectives shared by patients and caregivers that provide insights on treatment and what is needed.”
“While technically a rare disease, cystic fibrosis is one of the most widespread life-shortening genetic diseases in the Western World affecting 1 out of 3,700 US births or roughly 1,000 births per year,” said Brian O’Sullivan, MD, a pediatric pulmonologist and Director at the Cystic Fibrosis Center at UMass Memorial Medical Center in Worcester, MA. “More therapies are needed and it is important for those developing novel therapeutics to understand all aspects of this disease including what is now available, what is in the pipeline, and to keep in mind the patient experience and treatment burden.”
In addition to having current treatment and market information, Drug Market Info develops reports based on putting patients and caregiver’s front and center. “We are strong supporters of patient advocacy and our business model is built around donating a portion of our proceeds to the leading patient advocacy group in each therapeutic area we cover,” stated Mary Beth Cicero. Drug Market Info is extremely proud and privileged to be associated with the Boomer Esiason Foundation, and will be providing a donation for each report purchased to help CF patients. The Boomer Esiason Foundation is a dynamic partnership of leaders in the medical and business communities joining with a committed core of volunteers to heighten awareness, education and the quality of life for those affected by cystic fibrosis, while providing financial support to research aimed at finding a cure. To find out more information about this organization, please visit http://www.esiason.org.
“Every day researchers around the world are coming one step closer to finding a cure for cystic fibrosis. In the meantime, the CF drug pipeline ensures therapies are moving from the laboratory to the marketplace. Information on the CF market that highlights the opportunities and focuses on patients is needed to help this effort,” stated David Rimington, President of the Boomer Esiason Foundation. “We support the efforts of Drug Market Info for making this vital information available. We also applaud their efforts to support cystic fibrosis patients.”
Drug Market Info was founded by experienced pharmaceutical professionals who recognized the need for current, crisp, ready-to-use market information. Drug Market Info’s reports are unique in that they are organized in “presentation-ready PowerPoint format,” and centered around the concept of the three P’s – Patients, Perspectives and Potential with a strong focus on patient and physician perspectives.
About Drug Market Info
Have you ever just wanted to understand a disease – what it looks like, who gets it, what are the treatments, which products are used and why? Drug Market Info provides clinically accurate, up-to-date information on treating diseases such as Psoriasis, Psoriatic Arthritis, Cystic Fibrosis, Prostate Cancer and Parkinson’s disease, as well as how these markets are defined, sized and valued. Our market info reports deliver current, reliable information in a presentation-ready PowerPoint format at a reasonable price. In just minutes, you can download a customizable deck of slides covering everything you need to know about a disease, along with current treatment approaches and up-to-date U.S. market data trended for 5 years. Most importantly, only Drug Market Info provides patients’ insights on the disease and their treatment experiences. The Company proudly collaborates with leading patient advocacy groups so that every report purchased benefits patients with the disease.
Drug Market Info – The Patients’ Perspectives and the Info You Want Now!
Contacts
Drug Market Info
For Sales Information, please contact:
Mary Beth Cicero, Principal or Pamela Porter, Chief Business Officer
(855) DRUGMKT (378-4658)
sales@drugmarketinfo.com
Twitter: @DrugMarketInfo
or
For Media Inquiries, please contact:
Lisa Rivero, 855-378-4658
Lisa@drugmarketinfo.com
Cystic Fibrosis Fact Sheet
Looking for the facts about Cystic Fibrosis and the Cystic Fibrosis market? The following information comes from our Cystic Fibrosis Market Info™ Report which is now available for purchase and download on our website, Drug Market Info.
What is Cystic Fibrosis?
Cystic Fibrosis (CF) is a chronic, progressive and life-limiting genetic disease that impacts the lungs, digestive and reproductive systems, and sweat glands. CF affects the body’s ability to move salt and water in and out of cells, which causes production of thick mucus that blocks passageways. In the lungs, this mucus creates an ideal environment for bacterial growth, which results in frequent lung infections, breathing difficulties and eventually permanent lung damage.
Because the gastrointestinal (GI) system is also affected, children with CF often suffer with malnutrition, poor growth and general GI dysfunction. Other co-morbidities include sinus problems, diabetes, depression, osteoporosis and liver disease.
Most CF patients (88%) in the U.S are registered and treated through the Cystic Fibrosis Care Center Network, a part of the Cystic Fibrosis Foundation, consisting of 110+ treatment centers.
The Genetics of CF
The CF gene, located on chromosome 7, was first identified in 1989 and is thousands of years old. While over 1,800 mutations of this gene have been described, most are very rare. Over 80% of U.S. CF patients have a delta F508CFTR (∆F508 for short) mutation. This consists of a deletion of three base pairs in the gene, causing a loss of an amino acid in the CF transmembrane regulator protein.
CF is inherited in an autosomal recessive pattern, which means that two copies of the faulty gene must be present. Two CF carriers have a 1 in 4 chance of having a CF child.
Even with the exact same genetic mutation, CF affects patients differently and to varying degrees. Some have serious symptoms from birth; others have mild disease that shows up in teenage years.
It is believed that other unidentified genes modify the CF gene and determine disease severity.
Cystic Fibrosis: Diagnosis
Newborn screening for genetic disorders (including CF) is now performed in all 50 states, utilizing the immunoreactive trypsinogen test. As of 2009, about one-half of U.S. CF patients were diagnosed by newborn screening.
An easy, reliable test for diagnosing CF is the sweat test, which provides results in one hour. Genetic testing is available, but only the most common mutations are screened so a negative test does not always rule out CF.
Genetic testing is available, but only the most common mutations are screened so a negative test does not always rule out CF.
Cystic Fibrosis: Epidemiology
- 30,000 CF patients in the U.S.; 47% are over age 18.
- 10 million+ carriers in the U.S. or 1 of every 37 individuals.
- Incidence of CF is 1 of every 3,700 U.S. births or about 1,000/year.
- Caucasians represent over 90% of patients.
- Median life span is now the mid-30s, up from elementary school age in the 1950s.
- Males and females are equally affected.
- Over 90% of patients eventually die of lung disease.
Treatment
Close attention to and treatment of both respiratory and digestive problems has led to increased life span. Nearly all CF patients, starting as babies, require pancreatic enzymes (such as Creon® or Zenpep®), along with a high calorie, well-balanced diet, special vitamins and supplements.
Respiratory therapy consists of mucolytics (Pulmozyme® to thin and loosen mucus), hypertonic saline (to draw water into airways to move mucus), anti-inflammatories (to slow the rate of pulmonary decline) and inhaled bronchodilators (to widen breathing tubes), which all work to improve lung function, decrease infections and avoid hospital visits. In addition to medications, airway clearance therapy, with chest oscillator vests or other physical methods, is required to help move mucus out of the lungs. CF patients are often on chronic antibiotic therapy, such as tobramycin (TOBI) or Cayston® and other antibiotics as needed. In addition, a new CFTR-modulated therapy, Kalydeco® can help patients with a certain CF mutation. Many CF drugs are administered via a nebulizer and require 20-30 minutes for a treatment.
About 200 CF patients receive a bilateral lung transplant each year, representing 14% of all lung transplants performed annually. Bilateral lung transplants are usually performed in severe or advanced CF, and 5-year survival is 54%, which compares favorably with other transplant recipients.
Some Interesting Facts about Cystic Fibrosis
- CF has a very high treatment burden: Mean reported time/day spent on therapy is 108 minutes — even adults report family assistance is required.
- 98% of CF males are born with bilateral obstructed or absent vas deferens, rendering them infertile (correctable with Assisted Reproduction). Women are fertile.
- Even infants with no symptoms and children with normal lung function already have lung damage.
- CF patients are discouraged from interacting with other CF patients to avoid acquiring infections. The most common and troublesome is Pseudomonas aeruginosa.
Cystic Fibrosis: Research
There are 91 drugs in Phase 3 clinical development and over 100 in Phase 2. This includes drugs that target numerous CF disease features, such as airway modulation, airway surface liquid restoration, anti-inflammatories and antibiotics, as well as immune modulators and nutritional supplements. Over 20 Phase 1/2 trials utilizing various gene transfer agents have also been conducted.
What Do Patients Say About Cystic Fibrosis?
“With CF, people see a pump or an oxygen tank, and not a person. When my daughter went on a sleep-over, she would have to take pills, an oxygen tank and nebulizer.”
“You know, you get sick of being sick. You get fatigued easily, it’s hard sometimes to keep up with the work that’s needed for school and for life – you are just too tired. CF patients require a lot of calories and a lot of sleep.”
“This girl is on so much. She needs antibiotics, enzymes and insulin for CF, but she also had a transplant and needs anti-rejection drugs. All these drugs have side effects and she has developed high blood pressure and cholesterol problems. She’s only 19 years old.”
To learn more about the ‘Patients’ Perspective,’ Drug Market Info will gladly put you in touch with patients and clinicians who can share their experiences.
Every Cystic Fibrosis Market Info report you purchase generates a donation to the Boomer Esiason Foundation.
 The Boomer Esiason Foundation is a dynamic partnership of leaders in the medical and business communities joining with a committed core of volunteers to heighten awareness, education and the quality of life for those affected by Cystic Fibrosis, while providing financial support to research aimed at finding a cure.
The Boomer Esiason Foundation is a dynamic partnership of leaders in the medical and business communities joining with a committed core of volunteers to heighten awareness, education and the quality of life for those affected by Cystic Fibrosis, while providing financial support to research aimed at finding a cure.